
|
Proof of Concept Programs
Introduction
University discoveries can be identified (via investigator disclosure) and protected (via patenting), but due to the early nature of university technology, it is difficult to find an adopter and champion willing to develop and commercialize the invention. Discoveries and inventions made in the context of a research institution with support from federal research grants are typically immature when disclosed to the institutional technology transfer enterprise. Patent applications, or even issued patents, are not normally stand-alone, market-ready assets, nor are they the core of a viable development program. Furthermore, uncertainty with respect to technical feasibility, clinical feasibility, and market feasibility usually remain, when an invention is submitted to the institutional technology transfer office. Most technologies disclosed to technology transfer enterprises are not the result of a market-driven process, and thus are not immediately ‘transferrable’ to a private sector investor; the discovery, invention, or technology must evolve into a comprehensive development program to be conducive to significant capital investment and transfer to private investors. Public funding of these key validation steps has not historically been within the mission of most federal funding agencies – these public funds are generally allocated to projects that advance the creation of new knowledge and scientific principles, not validation of existing concepts.
Biomedical technologies generally involve greater development and commercialization risks compared to other university technologies in the physical, engineering, materials, and information science realms. Compared to other university technologies, biomedical technology is even further “over the horizon” due to longer patent prosecution risk, human safety and efficacy risk , risk of capital continuation, and reimbursement risk. Preclinical drug development, prototype development, assay development, and assay validation are not suitable aims for federal grant funding, so in many cases, biological discoveries lack a clear definition of the product, lead market application, and business/revenue model.
As a result of income generated from the commercialization of CU intellectual property, the CU TTO has created and supports Proof of Concept (POC) funding opportunities for research and business development. POC programs are intended to foster downstream capitalization of the technology development program and advance the project along its commercial roadmap. Given the unique challenges, risks, and costs in proving feasibility of novel biomedical technologies, coupled with the fact that roughly 2/3 of University of Colorado inventions are from the biomedical space, our investments have emphasized biomedical programs.
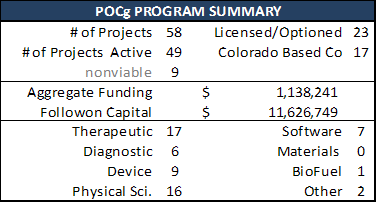
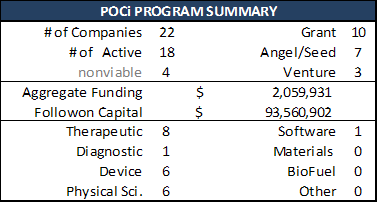
A summary of the various POC programs that have funded technology development at the University of Colorado is below:
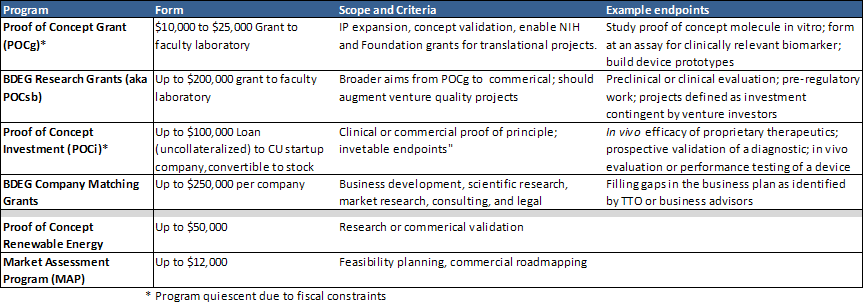
The success or failure of a seed feeding program should be measured relative to the goals of the Program. The goal of the University of Colorado’s program is to attract grant capital allocated by scientific merit and private capital from sophisticated investors; consequently, we track downstream funding events flowing to the programs supported by the various POC programs. Though several of the POC programs are relatively new and the projects they have supported are still incubating, substantial technical and commercial progress has been made with many of the programs. (see Silva, RS, Allen, DN, Traystman, R. Medical Innovation and Business, Spring 2009, p. 52-66).
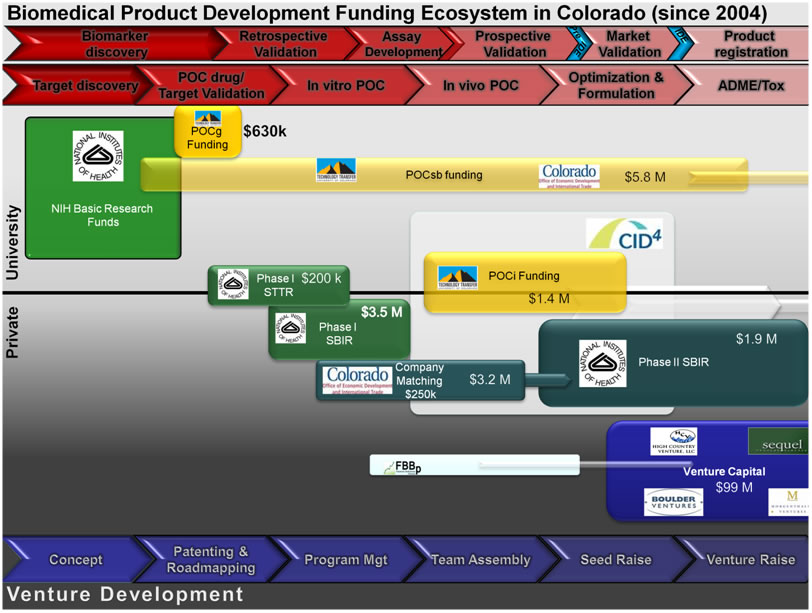
The schematic below shows the POC and BDEG funding programs in the context of the development continuum of a biomedical product and sources of follow-on funding (From NIH Clinical Translational Science Award, Public Private Partnership Key Function Committee Symposium. Bethesda, MD. February 2010).
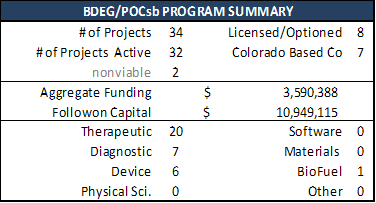
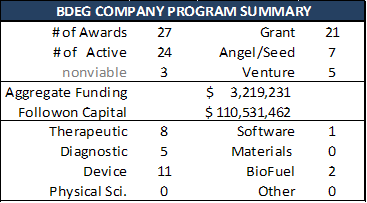
Scorecards
|